Command Palette
Search for a command to run...
Insight Into Insilico: The Leap, Dilemma and Breakthrough of the AI Pharmaceutical Star Company

Aging has troubled countless people of noble character and lofty ideals since ancient times. Whether it is the first emperor of Qin Shihuang, the ambitious Emperor Wu of Han, or the powerful Emperor Taizong of Tang, these great emperors have all left many stories of pursuing immortality. When the pointer of time fell on the second decade of the 21st century, with the intensification of the global aging problem, many companies tried to overcome this problem with digital technology. Among them, Insilico Medicine, a biopharmaceutical technology company driven by generative artificial intelligence, has quietly become a leader in the field of AI pharmaceuticals.
Insilico Medicine has built an artificial intelligence drug development platform mainly through self-developed generative adversarial networks (GANs), deep reinforcement learning (RL), pre-trained models (Transformer) and other machine learning technologies, focusing on identifying new targets and generating candidate drugs with molecular structures with specific properties, aiming to advance and accelerate the development of innovative drugs.
For a technology company like Insilicon Valley Technology with an international vision, this article will attempt to explore the important driving factors in the development history of Insilicon Valley Technology from several aspects, including technological development, team composition, and business ecology.
Technology: AI-driven mixed martial arts fighter in pharmaceuticals
Around 2013, with deep learning leading the AI revolution, AI pharmaceuticals began to attract pharmaceutical companies around the world. In 2014, Alex Zhavoronkov founded Insilico Medicine at Johns Hopkins University in Baltimore, USA. After eight years of hard work, Insilico Medicine finally successfully released a fully integrated end-to-end AI and robotic drug discovery system - Pharma.AI in 2022.In the field of AI pharmaceutical manufacturing, the entire process of target discovery, drug discovery, and clinical trial prediction has been completed.
AI technology driven, full process coverage
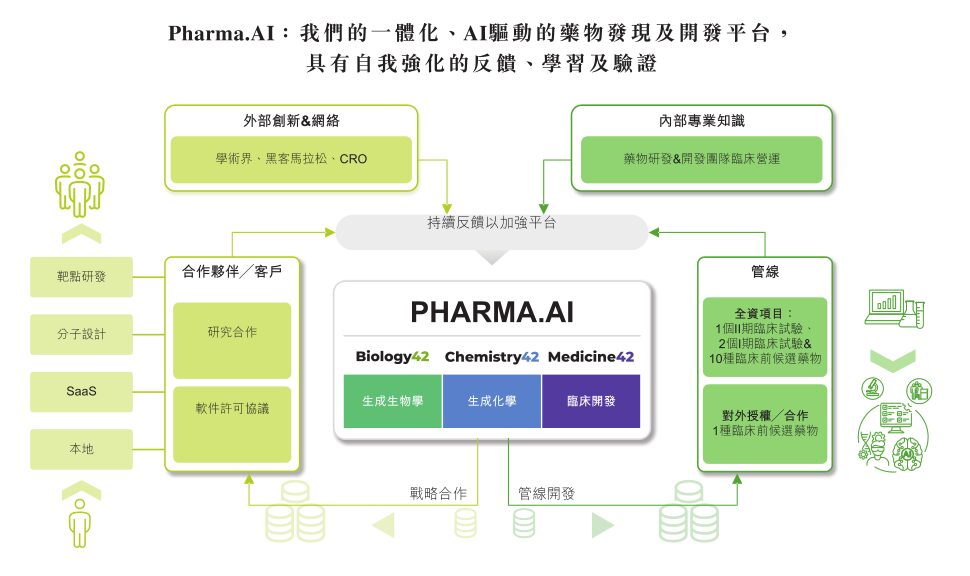
From a technical perspective, Pharma.AI is undoubtedly the core foundation of Insilicon Valley. According to the prospectus published by Insilicon Valley,The Pharma.AI platform mainly consists of three parts: Biology42, Chemistry42, and Medicine42.in:
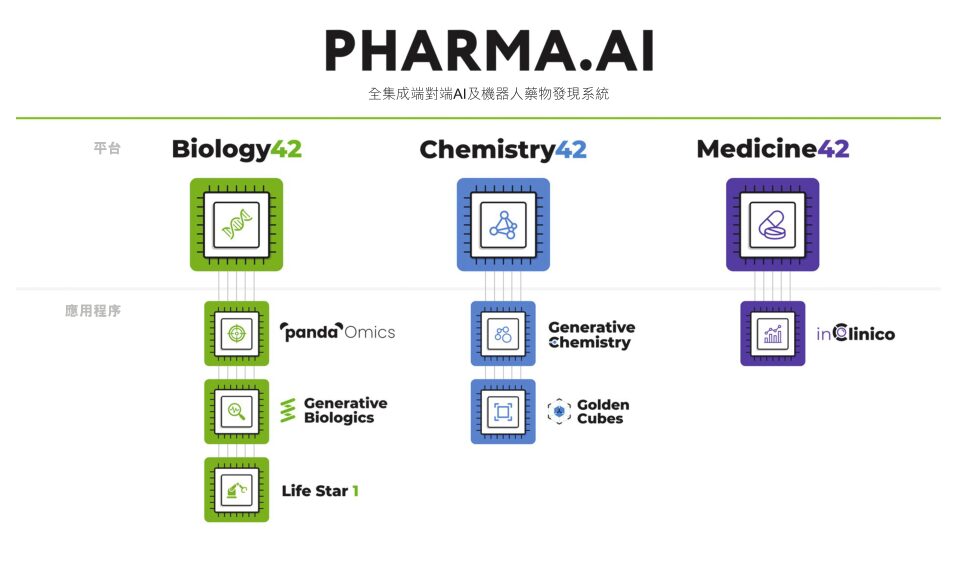
* Biology42:It includes three applications: PandaOmics, Generative Biologics, and Life Star 1, which are mainly used to identify and discover new targets;
* Chemistry42:It includes the generative chemistry platform Generative Chemistry and the drug off-target assessment module Golden Cubes. Its main function is to achieve rapid discovery and optimization of early drug compounds by combining structure-based drug design, ligand-based drug design, reinforcement learning, active learning and other technologies.
* Medicine42:It is mainly composed of the predictive clinical trial module inClinico, which can be used to model the success probability of mid- and late-stage clinical trials.
Although it seems very complicated, Insilico Medicine's technological accumulation did not happen overnight. The successful launch of Pharma.AI is the result of years of thinking and hard work.
In fact, in order to improve the efficiency of drug research and development, modern medicine has long proposed a target-based drug discovery method. If the disease can be arranged and combined with the targetable targets in the human body, it is possible to find treatments for certain diseases. However, the manual experimental method is not only time-consuming and labor-intensive, but also has a low success rate. Therefore, Alex Zhavoronkov boldly imagined that if hundreds of thousands of medical documents, research projects, and clinical trials were reviewed based on AI, potential disease mechanisms and therapeutic targets might be discovered. This is also the original research idea of PandaOmics, the first module of the Pharma.AI platform.
In simple terms,PandaOmics is a biological target discovery engine based on independent research and development.Based on a variety of biological research data and combined with deep learning technology, it can quickly and accurately identify potential targets and sort them according to the relevance of their effects, thereby extracting a series of potential targets for drug development.
In 2016, after two years of experience in the pharmaceutical research field, Insilico Medicine began to focus on drug design and officially started the research of Chemistry42. Chemistry42 is an automated machine learning platform for drug design.After finding the target, Chemistry42 can automatically design the corresponding protein drug structure based on a deep generation algorithm, and can test the affinity of various protein structures through calculations.
It is worth noting that Insilico Medicine did not officially launch PandaOmics and Chemistry42 until 2020, and launched inClinico, which can predict clinical trials, again in 2022. inClinico can be used for clinical risk assessment and portfolio classification. It can integrate biological data, scientific research papers, clinical data, scientist data, etc., and can predict the probability of success (PoS) of a single clinical trial.According to data from Insilico Medicine's internal evaluation, 5 out of the 7 prediction results inClinico provided for a world-renowned pharmaceutical company were accurate, with an accuracy rate exceeding 71%.
Also in 2022, Insilico Medicine combined Biology42, Chemistry42, and Medicine42 to create the Pharma.AI platform, which covers the entire process of using artificial intelligence to search for, verify, and test new drugs.
In addition, Insilicon Intelligence has also carried out a series of interesting explorations in different dimensions of depth and breadth:
* Using deep neural network (DNN) model to predict biological age
* Using genomic data to read out aging-related targets or physiological age
* Predict the response of the target to the drug
* …
AI+DD dual-wheel drive, self-developed pipeline progresses rapidly
Since 2018, the AI pharmaceutical industry has undergone a qualitative change. AI companies with R&D pipelines have gradually emerged, and the influence of artificial intelligence has begun to spread downstream. Insilico Medicine has keenly noticed the changes in the industry.We began to seek business transformation from an AI company to an AI+DD (AI+drug discovery) dual-wheel drive model.
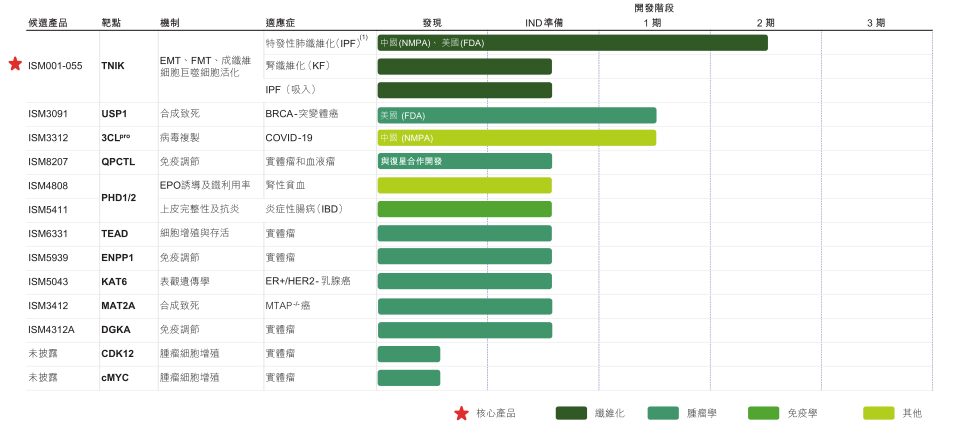
The prospectus shows that thanks to the continuous accumulation of the Pharma.AI platform in the early stage, Insilico Medicine began to quickly and effectively promote the fully self-generated AIDD pipeline after the strategic shift, and successfully identified a new anti-fibrosis target TNIK. Inhibiting TNIK may be able to solve fibrosis-related diseases. On this basis, Insilico Medicine successfully launched clinical candidate drugs and core products ISM001-055.
ISM001-055 is a potential first-in-class small molecule inhibitor independently developed by Insilico Biologics.It completed the identification through the biological target discovery engine PandaOmics, and then used the generative chemistry platform Chemistry42 to generate and design a new molecular structure for the new target, mainly for the treatment of fibrosis-related diseases such as idiopathic pulmonary fibrosis (IPF). The entire process, from disease hypothesis conception to Phase I clinical trials, took about 30 months. Using traditional methods, it usually takes at least 6 years.
In early 2023, INS018_055 was tested in multiple single-ascending dose (SAD) and multiple-ascending dose (MAD) cohorts in Phase I trials in New Zealand and China. The international multicenter Phase I study achieved consistent results, demonstrating the good safety, tolerability and pharmacokinetic (PK) characteristics of INS018_055, which promoted the initiation of Phase II studies.
In April 2023, Insilico Medicine launched a multicenter, randomized, double-blind, placebo-controlled Phase IIa clinical trial to evaluate the safety, tolerability, PK characteristics and efficacy of ISM001-055 in China. At the end of the same year, it also obtained IND approval from the US FDA for the Phase IIa clinical trial of ISM001-055 for the treatment of idiopathic pulmonary fibrosis.
Although INS018_055 is being rapidly developed, Insilicon Valley's accumulation goes far beyond this.As of the end of 2021, Insilico Medicine has reached cooperation with 10 of the top 20 pharmaceutical companies in the world.We began to use our own technology and development capabilities to accelerate drug research and development.
also,As of June 2023, Insilico Medicine has established a diversified, fully internally generated pipeline of 31 projects covering 29 drug targets.A rich pipeline has been created in therapeutic areas with huge unmet needs, such as oncology, immunology, and fibrosis.
Multi-dimensional exploration, innovation
Relying on its cutting-edge practices in the field of AI, Insilicon Valley has also explored multiple fields to strengthen its leading position in the application of AI technology from different levels.
As early as 2021, Insilico Medicine announced a collaboration with agricultural technology company Syngenta Crop Protection. Insilico Medicine will use AI and deep learning to innovate new active ingredients more efficiently and accelerate the invention and development of new and more effective crop protection solutions for growers.
In 2022,Insilicon Valley held the "Life Star - Sixth Generation Intelligent Robot Laboratory Launch Ceremony" at Suzhou Biomedical Industrial Park (BioBAY).The world's first fully automated robotic laboratory with AI-assisted decision-making was officially unveiled. This intelligent robotic laboratory focuses on target discovery, compound screening, personalized drug development, and translational medicine research, aiming to efficiently transform the drug discovery process, comprehensively improve the success rate of drug research and development, and accelerate the satisfaction of unmet clinical needs.
Under multiple influences, Insilicon Valley Medicine is continuously exploring the possible applications of intelligent automation in data processing and new drug research and development, using next-generation artificial intelligence and robotics technologies to transform new drug research and development, and injecting new impetus into cross-industry integrated innovation and digital drive.
Team: Dual CEO organizational structure, recruiting global talents
Cutting-edge companies often have advanced management models, and this is exactly the case with Insilicon Valley.Insilico Medicine adopts a unique dual-CEO organizational structure, with Alex Zhavoronkov, who has strong digital and Internet genes, responsible for AI technology iteration, and Ren Feng, who has been deeply involved in drug discovery for many years, leading the drug discovery business. It has also attracted more than 150 academic and industry collaborators around the world.Perhaps, this is precisely the "secret" of Insilicon Intelligence to lead the industry.
Founder Alex Zhavoronkov

Alex Zhavoronkov, the founder of Insilicon Intelligence, was born in Riga, the capital of Latvia, in 1979. Throughout his adolescence, Alex Zhavoronkov witnessed the fading Soviet empire and the turbulent society, which also gave him more layers of thinking about human aging.
"We are given life, to grow, to thrive, to develop careers, to reproduce, and then in an instant we lose everything we created and die. Why does this happen?" In order to find the answer, Alex Zhavoronkov studied a lot of knowledge about eating habits, exercise, sleep regulation and other fields in high school, but his confusion was not completely solved.
The development of computer science gave Alex Zhavoronkov hope. He obtained bachelor's, master's and doctoral degrees in computer science, physics and biotechnology from Queen's University in Canada, Moscow State University and Johns Hopkins University. After graduation, he worked as a consultant in a consulting company, providing services to biotechnology companies.
With years of continuous accumulation and accurate grasp of the trends of the times, Alex Zhavoronkov started to link AI with biology and chemistry at a very early stage and successfully founded Insilicon Intelligence.
In order to prove to the outside world the capabilities of AI in the pharmaceutical industry,In 2019, Alex Zhavoronkov published a paper titled "Deep learning for rapid identification of effective DDR1 kinase inhibitors" in the journal Nature Biotechnology, introducing how Insilico Medicine used its self-developed AI system to conceive and design new drug molecular structures, and demonstrated the success of this drug in mouse testing experiments.
The system takes only 21 days to design a molecule, and the total time for design, synthesis and verification is about 46 days. This achievement confirms the ability of AI to accelerate drug discovery and also makes Insilico Medicine well-known in the industry.
Dr. Ren Feng, a leader in the pharmaceutical industry

Among the many technology challengers disrupting traditional industries, Dr. Feng Ren, Co-CEO and Chief Scientific Officer of Insilico Medicine, stands out with his deep experience in the pharmaceutical industry.Not only does he have more than 11 years of experience in small molecule drug research and development at the multinational pharmaceutical giant GlaxoSmithKline, where he held important leadership positions, he also played a core role in MediSci's entrepreneurial journey, helping the company become a pioneer in the CRO field on the Science and Technology Innovation Board.
Dr. Ren Feng, who has accumulated 14 years of valuable experience in the field of new drug research and development, has deeply realized the inefficiency of the traditional research and development model. He realized that in order to break through the status quo, new tools and methods must be explored. However, in the context of the era when AI technology is in the ascendant, even senior experts in the traditional pharmaceutical industry like Dr. Ren Feng are faced with the confusion of choice. It is this desire for innovation and exploration of the future that ultimately led him to embark on a new journey of applying artificial intelligence technology to drug research and development.
In the summer of 2020, when Ren Feng first communicated with Alex Zhavoronkov on the phone, he was planning his transformation. He was very interested in emerging technology-driven drug development, but he was "half-believing" about whether AI could really make a drug. In September 2020, he joined Insilico Medicine as a consultant, completed a lot of background checks, and tried the company's artificial intelligence technology platform. After experiencing the process of Insilico Medicine's first anti-fibrosis project from target identification to the discovery of preclinical candidate compounds, he decisively chose to join.
When Ren Feng officially joined the company in February 2021, the Insilico Medicine China team consisted of only three people. Under his leadership, the company quickly established a drug research and development team of nearly 150 people in more than two years.This is also the largest team of Insilico Medicine in the world. As a result, Insilico Medicine, which is driven by artificial intelligence and drug research and development, has entered a period of rapid development.
Insilico Medicine Scientific Advisory Board
As a technology-leading technology company, Insilicon Valley has accumulated very deep academic resources around the world and established a scientific advisory committee that brings together a large number of the world's leading scientific research leaders.
For example, Charles Contor, a member of the National Academy of Sciences and chief scientist of the Human Genome Project, is a member of the Scientific Advisory Board. He once said, "In drug development, speed is everything. At least 90% of the cost associated with approving a drug for human use is in the late stages of clinical trials. With its AI-driven universal drug discovery system, Insilico enables researchers to rule out failed methods faster and earlier at many stages of the drug discovery process and before clinical trials, so as not to be too late."
Michael Levitt, the 2013 Nobel Prize winner in Chemistry and professor at Stanford University, believes that“There are many AI pharmaceutical companies. Some teams try to discover targets, while others conduct chemical research. There are very few companies like Insilico that can cover the entire process.”
Standing on the shoulders of a group of academic giants, Insilicon Valley has taken the lead in AI technology and created a unique landscape.
Business: With three major business models, why is the road to listing so bumpy?
Insilico Medicine is particularly noteworthy for its significant contributions in the field of generative AI for drug discovery and development.
From the perspective of business model,Insilico Medicine combines the three mainstream AI pharmaceutical business models: AI+SaaS, AI+Biotech and AI+CRO.Through Insilicon Valley, we can have a general understanding of the commercialization status of the domestic AI pharmaceutical industry.
Judging from the results, the upper limit of SaaS software licensees is not high, and now only accounts for about 5% of Insilicon Valley's annual revenue. In terms of new drug research and development in external cooperation, Insilicon Valley currently mainly relies on cooperation with Fosun and Sanofi. Although this part of the income is considerable, it is often too dependent on a few large customers, and the down payment is not high.
In 2022, Insilico Medicine signed a major cooperation agreement with Sanofi, under which Sanofi will use Insilico Medicine's Pharma.AI drug discovery platform to advance the development of candidate drugs based on no more than six innovative targets.This contract worth $1.2 billion received only a down payment of $21.5 million, with a down payment ratio of less than 1.8%.
In terms of self-developed pipeline development, 29 of Insilicon Valley's 31 current projects are completely self-developed, equivalent to half of the number of projects of Johnson & Johnson, a large pharmaceutical company. This also means that this part of the business still swallows up most of Insilicon Valley's R&D expenses at this stage, and the cost and risk remain high. The prospectus shows thatInsilicon Valley Smart Technology suffered losses of 940 million yuan and 1.59 billion yuan in 2021 and 2022 respectively, with losses exceeding 2.5 billion yuan in just two years.This brings huge uncertainty to the company's future development.
Looking at Insilico Medicine’s exploration in the field of AI pharmaceuticals, although AI can save time and economic costs in early research and development, which accounts for 3% of the entire drug development process, the efficiency advantage has dropped sharply since the clinical stage, which also shows that AI’s empowerment in modern pharmaceutical processes is still limited.
Despite this, Insilicon Valley Technology, which uses AI as its main entry point, is still sought after in the capital market.
Since its establishment,Insilicon Valley Silicon has gone through seven rounds of financing, with a total financing amount of US$407.5 million, equivalent to RMB 2.95 billion.Among them are Qiming Venture Partners, Sinovation Ventures, Sequoia Capital, Fosun Pharma, WuXi AppTec, Boston Investment Group B Capital Group, and Saudi Aramco's diversified venture capital fund Prosperity7 Ventures. The post-investment valuation has reached US$895 million, or approximately RMB 6.47 billion.
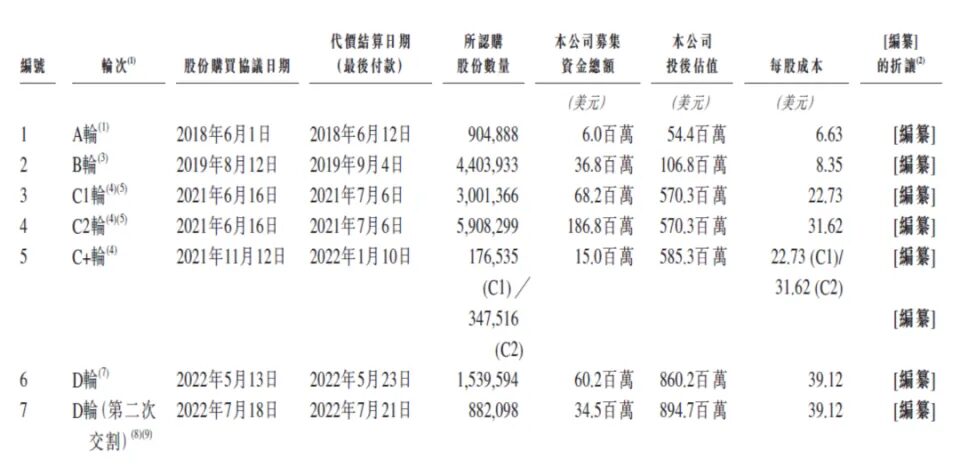
It is worth noting that WuXi AppTec plays an important role in the development of Insilicon Valley.
In 2017-2018, due to investors’ low confidence in generative AI, Insilico Medicine had great difficulty in raising funds in the early stage. It was not until the emergence of Li Ge, the founder of WuXi AppTec, that Insilico Medicine was helped.
In June 2018, WuXi AppTec Venture Capital Fund led a new round of strategic financing for Insilico Medicine. Both parties expressed their intention to closely integrate Insilico Medicine's AI technology with WuXi AppTec's experimental capabilities.
To this day, Alex Zhavoronkov still keeps a photo of Li Ge in his office to thank him for his help. In 2019, Alex Zhavoronkov moved the headquarters of Insilicon from the United States to Hong Kong, China, which made it even closer to China.
However, before Insilicon Intelligence, which had “moved eastward”, could take a piece of the domestic market,It was already involved in a hot US-China technology war: American startup, Chinese co-CEO, American shareholders, Chinese headquarters...Being entangled in the game between the two major powers, from a positive perspective, there is considerable room for mediation, allowing the country to have its cake and eat it too. However, from a negative perspective, the country may be caught in a dangerous situation of being attacked from both sides.
In this ever-changing and treacherous international competition, is the "hybrid" Insilicon Warehouse hot or hot potato? Is it sought after by investors or is it kept at a distance? Before the final conclusion is made, it is probably the most urgent thing for Insilicon Warehouse to find new financing channels as soon as possible, which also forces them to launch an IPO at this point. You know, for traditional pharmaceutical companies, the company must have at least 2-3 pipelines entering the second phase before it dares to consider listing.
In January 2024, the ill-fated Insilicon Valley Technology's Hong Kong stock prospectus expired.This has once again triggered the industry to think about whether AI pharmaceutical manufacturing is feasible. On March 27, 2024, Insilicon Valley submitted its second listing application to the Hong Kong Stock Exchange.Go all out to become the "first AI pharmaceutical stock". According to the main board listing process officially announced by the Hong Kong Stock Exchange, after a company submits its listing application, it will receive the first round of opinions from relevant departments within 15 working days.
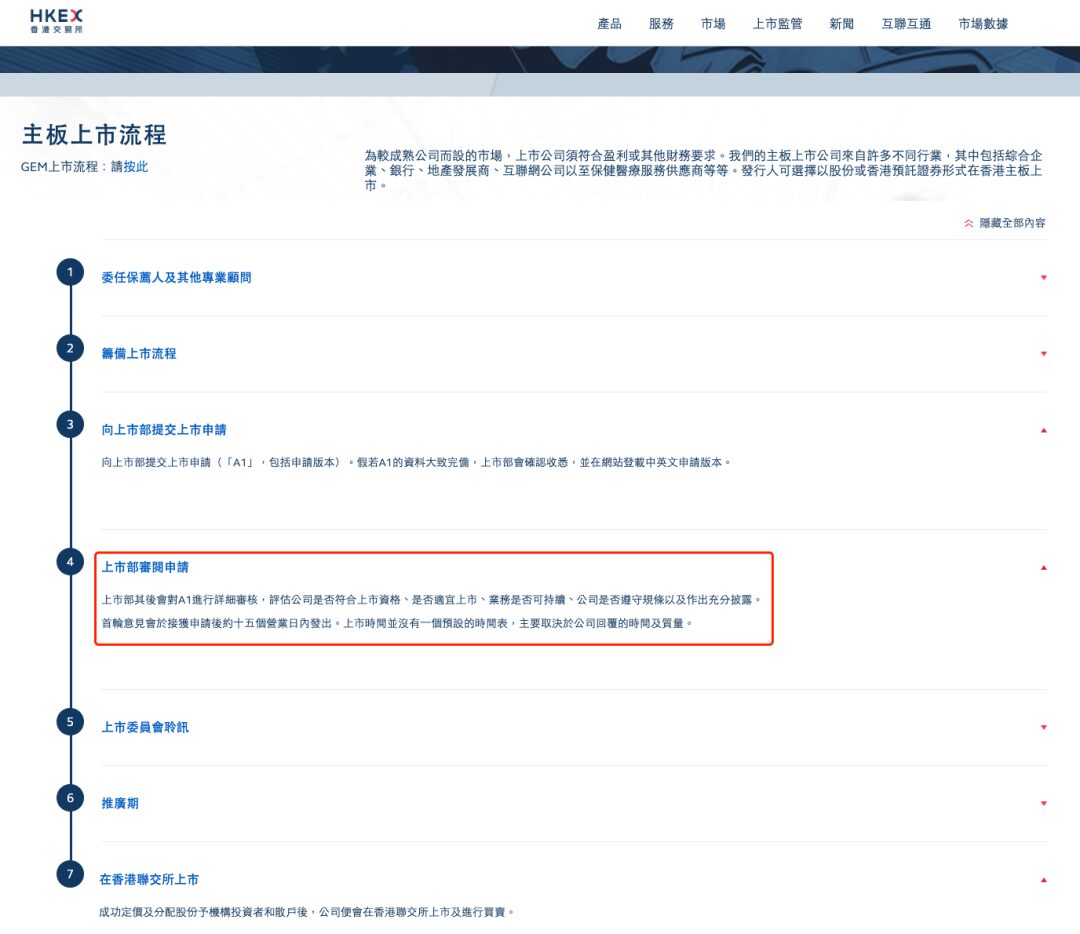
Although the main board of the Hong Kong Stock Exchange supports multiple listing applications, each submission of information requires a lot of manpower, material and financial resources. Whether Insilicon Valley's second round of listing on the Hong Kong Stock Exchange can be successful, the industry and upstream and downstream capital are paying as much attention to the result as Insilicon Valley itself.
Thinking: Facing multiple challenges, can we stand out?
It is obvious to the naked eye that Insilicon Valley's predicament will not be completely resolved in the short term.
In addition to the US government's step-by-step pressure on China's AI industry, as a foreign company, Insilico Medicine also faces huge restrictions in using Chinese biological data, which will undoubtedly hinder the development of Insilico Medicine. Perhaps it is precisely because of the consideration of the above issues thatIn February 2023, Insilicon Valley’s AI R&D center in Abu Dhabi, the capital of the United Arab Emirates, was officially put into operation.
As it gradually retreats from the Chinese and American markets, Insilicon Valley is in urgent need of opening up new outlets around the world, and the "rich" Middle East market has undoubtedly become a potential destination. This not only means that Insilicon Valley has made outstanding achievements in globalization, but also implies that Insilicon Valley is destined to drift around the world forever.
While Insilico Medicine is constantly looking for a way out, changes in the field of AI pharmaceuticals are also quietly taking place.
From 2012 to 2015, well-known international AI pharmaceutical companies such as Exscientia, Recursion, BenevolentAI, Relay, and Atomwise were established. Following closely, domestic AI pharmaceutical companies such as Jingtai Technology, Iceland Stone, and Bio-Bio emerged from 2015 to 2018. These companies are based in the Chinese and American markets, and their actual attractiveness in terms of capital, technology, and talent has far surpassed Insilicon Warehouse, and they are already showing signs of surpassing Insilicon Warehouse in terms of strength.
For example, on November 30, 2023, Jingtai Technology submitted a prospectus to the Hong Kong Stock Exchange. As of July 2021, Jingtai Technology's valuation after the D round of financing has reached US$1.968 billion, far exceeding the US$894.7 million when Insilicon Valley completed its D round of financing in July 2022.
In a recent interview, Alex Zhavoronkov made it clear thatAs a company founded by a Latvian-Canadian, headquartered in Hong Kong and New York, with customers mainly from mainland China, Insilicon Valley has once tasted the dividends brought by its international genes, but is now being squeezed by the trend of de-globalization.Drug development is a global challenge that transcends national borders, but the continued tension in the geopolitical situation is bringing huge risks. Not only that, Alex Zhavoronkov is also anxious that his physical condition is getting worse every day, and there is not much time to wait.
So, how should Insilicon Smartech break out of the situation, which is constrained by the international situation and the current status of Hong Kong stocks? Perhaps we can draw on the experience of predecessors to make some guesses.
As the leader of the four AI unicorns, SenseTime's listing process is also full of twists and turns. On August 27, 2021, SenseTime officially submitted its listing application to the Hong Kong Stock Exchange and was approved by the Hong Kong Stock Exchange at the end of November. However, in the early morning of December 11, the U.S. Treasury Department suddenly included it in the "Military-Related Enterprise List", prohibiting U.S. investors from investing in SenseTime. In an emergency, SenseTime decisively announced the postponement of the global offering and listing, and quickly adjusted the cornerstone investors, and finally successfully listed on December 30.
The reason why the adjustment was so rapid is due to SenseTime's absolutely strong technical strength. Similarly, if Insilicon Valley wants to reverse its decline as soon as possible, it must speed up the progress of its self-developed pipeline and demonstrate its strong strength in some key disease areas.
Obviously, for Insilicon Intelligence, going public is still imperative. But at present, the continued tight cash flow, the rising star's step-by-step pressure, and the huge impact of the geopolitical situation have increased the difficulty and uncertainty of this matter at different levels. Can it win the medal of honor of "the first AI pharmaceutical stock"? Can it ensure that the myth will not be destroyed after successfully winning the medal? Let's wait and see.
Reference articles:
1.https://www.fxbaogao.com/view?id=3950893
2.https://mp.weixin.qq.com/s/z0Jrhux6an1QKpQeSnTL4Q
3.https://mp.weixin.qq.com/s/pmq0Q5AWAZEi3tSq4dxygw
4.https://mp.weixin.qq.com/s/Z3r-rvXZ6KMWBJ-Uv9B7aQ
5.https://mp.weixin.qq.com/s/_N4szkv16mMcrWkg_FEHtA
6.https://baijiahao.baidu.com/s?id=1772180224197472503&wfr=spider&for=pc
7.https://new.qq.com/rain/a/20230630A08URE00
8.https://zhuanlan.zhihu.com/p/640650764