Command Palette
Search for a command to run...
Ainnova Tech Develops a 3-second Detection Platform With an Accuracy Exceeding 90%. Its Clinical Trial Plan Has Received FDA guidance.

From the description in the Yellow Emperor's Classic of Internal Medicine that "amblyopia is like seeing nothing," to modern medicine's understanding of the risk of retinal blindness, humanity's exploration of the relationship between eyes and health has never ceased. Among them, retinal disease, particularly diabetic retinopathy and retinitis pigmentosa, is one of the leading causes of blindness.
The Global Burden of Disease Study (GBD Study 2021) published by The Lancet shows that in 2021, there were 529 million people living with diabetes worldwide, with an age-standardized prevalence of 6.11% per year. Among these, the global prevalence of diabetic retinopathy was approximately 40% per year. Consequently, millions of people suffer from moderate or severe diabetic retinopathy, severely impacting their quality of life and even preventing them from working properly. Nearly 4 million people suffer from blindness as a result.
In fact, if fundus examination is performed promptly and an accurate diagnosis is made, these cases can be discovered and cured in time.Although diabetic retinopathy has become the leading cause of blindness among working-age adults worldwide, the diagnosis and treatment of this disease is still immature, with a diagnostic error rate as high as 49%.
Based on this big dilemma, in 2020,Ainnova, a health technology company based in Nevada with dual headquarters in Costa Rica and Houston, has emerged to create a powerful tool that can assist doctors.This will effectively reduce the error rate of diabetic retinopathy, provide patients with accurate information, and truly improve their lives.
As of July 15, 2025, Ainnova and its partners have completed a pre-submission meeting with the U.S. Food and Drug Administration (FDA), and its clinical trials will be conducted exclusively in the United States, focusing on diabetic retinopathy.
Company website:
https://ainnovatech.com/index.html
Retina: A redefined "whole-body health database"
A retinal exam shouldn't just focus on the eyes; it should serve as an early warning radar for systemic illnesses. The retina is incredibly precise. This transparent membrane, nestled against the inner wall of the eyeball, serves as the "photosensitive film" of the visual system, converting light signals into nerve impulses that form vision. It also serves as a barometer of overall health. Its vascular distribution is deeply connected to the systemic microcirculatory system. Diabetes can cause retinal blood vessels to leak, while hypertension can thin and harden them. Even cardiovascular and liver abnormalities can leave traces here.
A shocking set of data shows that more than 30% of diabetic patients will suffer from retinopathy, but treatment is delayed due to the lack of symptoms in the early stages; more than one million cases of blindness are caused by undetected retinopathy each year worldwide, and 95% could have been avoided through early diagnosis.Seeing the potential of this "window," Ainnova is determined to use AI to make the retina an "emerging vital sign" that can be quantified and interpreted.
Vision AI Technology Matrix: Breakthroughs from the Lab to Community Pharmacy
From technical principles to practical applications, Ainnova Tech completed a "triple jump" in 5 years. It launched the disruptive Vision AI platform.This technology matrix, built around computer vision and deep learning, has formed a full-chain capability covering "detection-assessment-intervention." Retinal image-based AI diagnostic technology has transformed early disease diagnosis from a "hospital privilege" to a "community norm."
Core layer: retinal image parsing engine
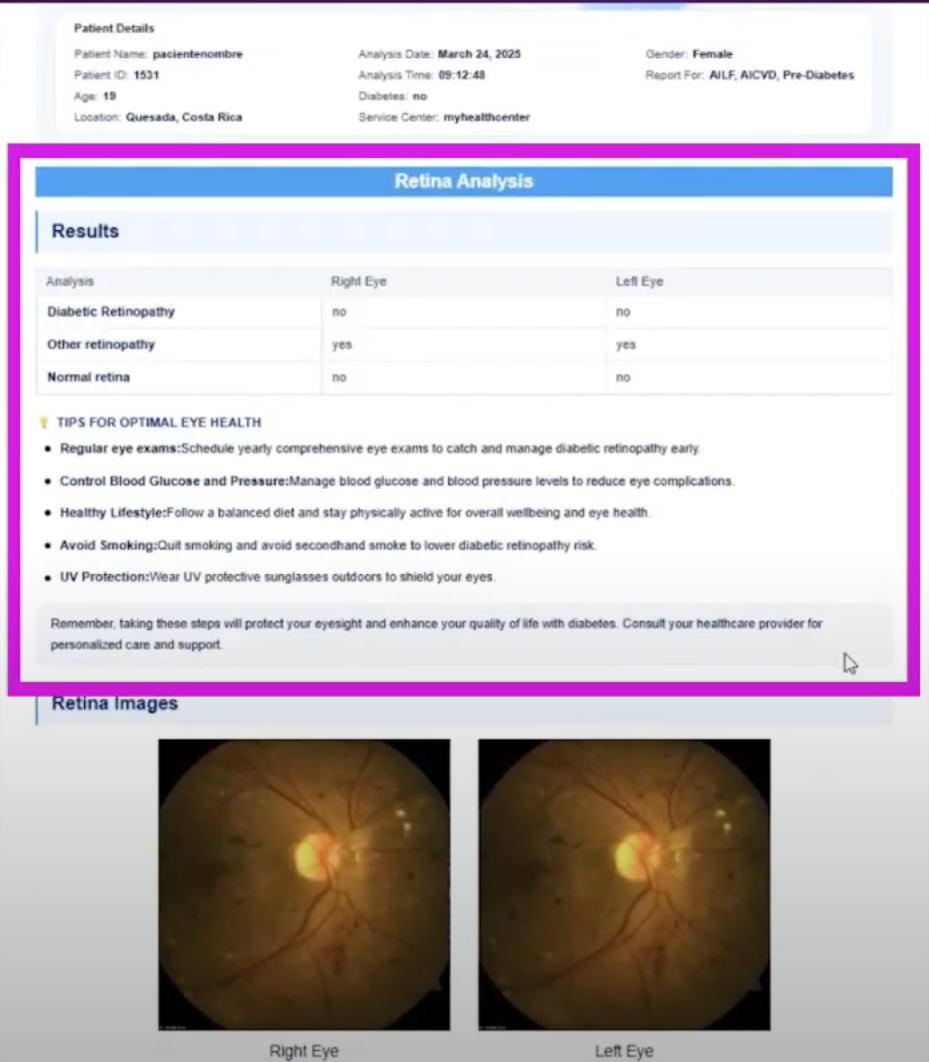
Based on convolutional neural networks (CNN) and Transformer architecture, the Vision AI platform can process high-resolution images captured by non-mydriatic fundus cameras.Complete the classification of 6 types of eye diseases including diabetic retinopathy and hypertensive retinopathy within 3 seconds.Generate clinical reports including lesion location and severity to support rapid referral by primary care physicians.
Extension layer: Multi-system risk assessment model
Extending from retinal features to overall health, a single non-invasive fundus scan can complete a multi-dimensional health assessment in seconds, building an AI diagnostic matrix covering "eyes-heart-liver-kidney-metabolism" and providing diverse diagnoses, including:
* Diabetic retinopathy detection:Vision AI uses high-resolution retinal images captured by a non-mydriatic camera and employs advanced deep learning algorithms to identify signs of diabetic retinopathy (DR), hypertensive retinopathy, and other conditions. It categorizes the severity of the condition and generates clinical reports to guide timely specialist referrals. The project has been clinically validated and is currently in the FDA submission process, aiming to become an "AI ophthalmologist" for primary care clinics.
* Cardiovascular risk prediction:Based on the Apollo Hospital-validated model, the system analyzes age, blood pressure and other data to predict an individual's 10-year risk of coronary artery disease (CVD) or cardiac events, providing personalized risk scores and care recommendations with an accuracy exceeding 90.%.
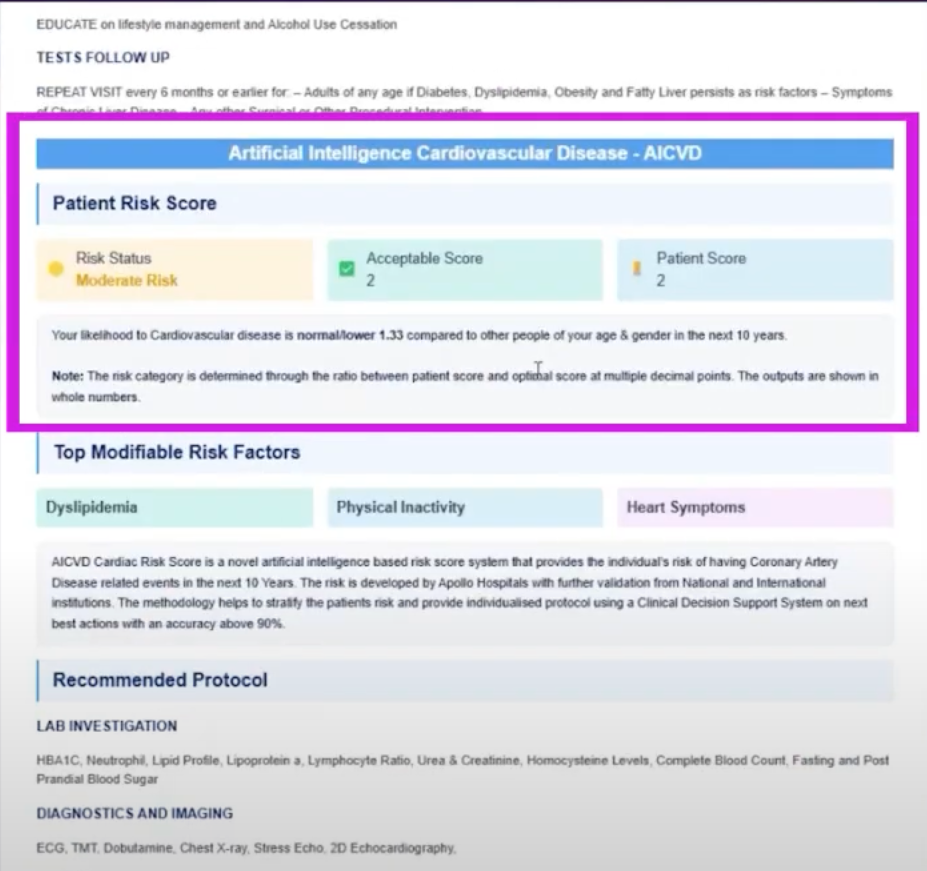
* Metabolic and organ risk assessment:It includes prediabetes risk (AUC is 0.86), liver fibrosis detection (AUC is 0.88), and chronic kidney disease progression prediction (accuracy exceeds 85%), realizing "one picture, multiple checks".
Implementation layer: health tools across multiple systems
In a community clinic in New Delhi, India, doctors use Vision AI to complete dual retinal and cardiac risk screening for diabetic patients within 5 minutes; in a chain pharmacy in Mexico City, pharmacists use the system to generate a "retinal health report" for customers.This tool, originally designed for ophthalmology, has become a "hub" for preventive medicine across multiple systems.In addition, experts and scholars in the industry have also expressed their high praise for this platform.
Inclusive healthcare: Free screening starts at pharmacies
"Most diabetic patients don't seek medical attention until their vision becomes blurred, by which time the optimal intervention period has passed." This is a core pain point identified by the Ainnova team during their global research. Ainnova believes that health screenings shouldn't be a "privilege" reserved for a select few. They repeatedly emphasize that diabetics and those with a family history of the disease should undergo a retinal exam at least annually, but even those without a history of the disease can benefit from this test.
To this end, the company launched a revolutionary preventive healthcare model in July 2025: in Latin America, diabetic patients can receive free retinal risk screening at the nearest pharmacy, without the need for pupil dilation or specialist doctors, and the entire process is painless and burden-free. This vision of "universal healthcare" gives technological breakthroughs a warmer practical background.
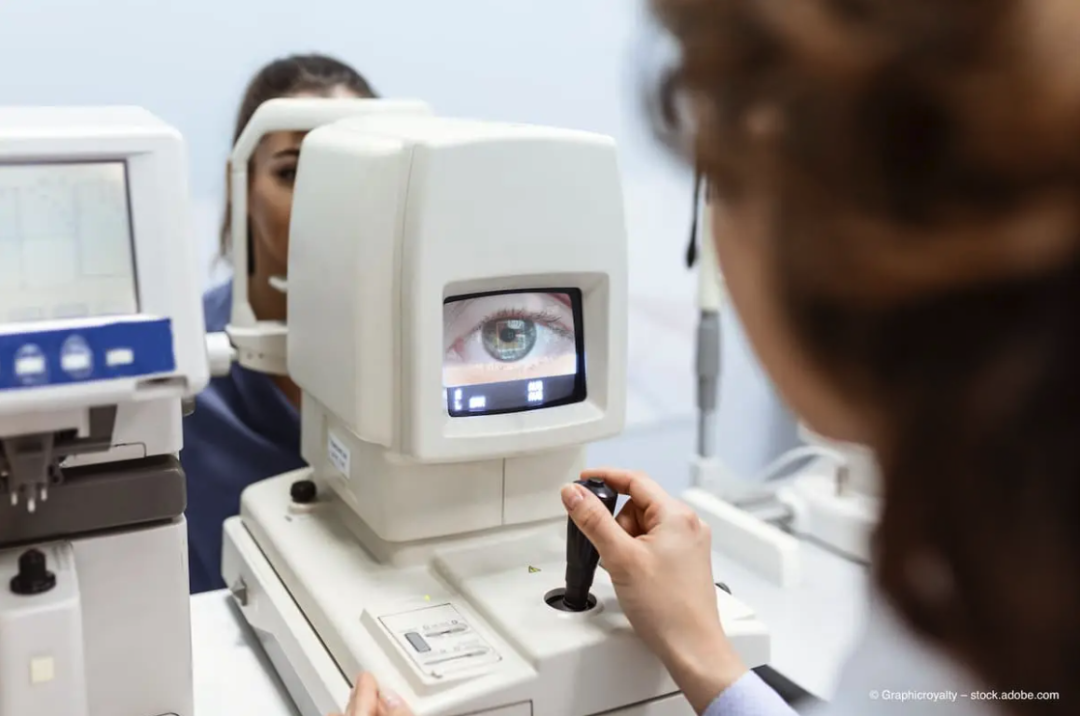
"We want to make retinal examination as easy as taking blood pressure."As early as May 2023, Ainnova teamed up with Costa Rican manufacturing services company ITEK to create an AI-driven, cost-effective imaging device for early disease detection. The device captures fundus images through a retinal camera to diagnose various eye diseases such as glaucoma, diabetic retinopathy, and macular degeneration. The collected images will be seamlessly transmitted to the Vision AI network platform.The platform uses advanced artificial intelligence algorithms to automatically analyze images, helping healthcare providers quickly and accurately identify potential health problems.
The underlying logic of this model is "reconstructing the medical landscape." Ainnova integrates ecosystem partners such as pharmacy chains, insurance companies, and medical clinics, shifting screening locations from hospitals to high-traffic, everyday settings. In Costa Rica, local pharmacy screening coverage increased to 80% within three months. In Brazil, high-risk patients identified by AI are referred to specialists within 48 hours, accelerating intervention time by 6-12 months.
The "economical imaging device" developed in collaboration with ITEK has successfully reduced the cost of fundus cameras by 60%, making it easily available to grassroots institutions in remote areas. This dual innovation of "technology + model" is suitable for non-specialized medical settings such as pharmacies and community clinics. The "free screening model" implemented in Latin America has broken down the barriers of "expensive and difficult early screening"—patients can complete the test at their nearest pharmacy, and high-risk individuals are referred to specialists within 48 hours, shortening intervention time by an average of eight months.
“We are very excited to be working with ITEK on this groundbreaking project. This camera has the potential to revolutionize the way healthcare is delivered, and we are confident it will make a real difference to the lives of patients around the world,” said Vinicio Vargas, CEO of Ainnova Tech.
Milestones and collaborations: From FDA guidance to a global healthcare ecosystem
Ainnova's technological breakthroughs have always been driven by deep collaboration. At the end of 2024, a joint venture and licensing agreement with Avant Technologies gave rise to Ainnova Acquisition Corp. (AAC), accelerating the commercialization of its Vision AI platform and multi-function retinal camera.In 2025, the two parties further plan to establish a new company to extend their layout from diabetes testing to the treatment field, and expect to drive explosive growth in revenue in 2025-2026.
Furthermore, Ainnova's rapid growth is inseparable from breakthroughs at key milestones. Previously, the company, leveraging its technological prowess, was recognized as one of the "Top 10 Most Promising Companies in the U.S. for the 2024 Entrepreneurship World Cup (EWC)."In July this year, its pre-submission meeting with the FDA once again became the focus of the industry: As a global medical regulatory benchmark, the FDA provided core guidance for its diabetic retinopathy clinical trials, including clinical program design, required clinic types, the number of evaluating doctors, etc., paving the way for the standardized clinical application of Vision AI.
AAC holds global licensing rights (including the United States) to the Ainnova technology portfolio. Since the FDA regulates the development of drugs and medical devices, the success of Ainnova's clinical trials is critical to the promotion of this technology portfolio in the United States. Entering the US market has huge commercial potential, and this early collaboration with the FDA ensures AAC can quickly and reliably launch proven products.
According to the latest news reports, on July 29, Ainnova revised the clinical trial protocol for its Vision AI platform for the early detection of diabetic retinopathy based on FDA comments. The new protocol will be resubmitted to the FDA for review to reduce costly errors in trial development. In addition, the Ainnova clinical development team met with the contract research organization Fortrea and submitted the updated protocol documents. If approved, the protocol will lay the foundation for the initiation of a new clinical trial.
Vinicio Vargas stated that the company's goal is to obtain FDA 510(k) clearance to market the technology in the U.S. and provide a transformative solution. The pre-submission meeting is expected to finalize the clinical trial budget soon, and the estimated cost will become clearer as the protocol is refined and the process progresses.
"This collaboration is not a simple technology licensing, but a collaboration in the medical ecosystem," said Chris Winter, CEO of Avant. "We are working with Ainnova to screen diabetic patients to identify early signs of diabetic retinopathy, and we are exploring some promising opportunities to participate in global diabetes treatment development."

Expanding the boundaries of technology: from ophthalmology to neurodegenerative diseases
Today, Ainnova's presence extends across the Americas, with offices in Houston (USA), Costa Rica, and Mexico. The team stands ready to respond to global demands for medical innovation. From algorithm iteration in the laboratory to precise diagnoses in the clinic, Ainnova is redefining the speed and precision of medical testing with AI.
However, Vision AI's ambitions extend beyond its current scope. Ainnova is expanding its technological boundaries in two key areas:The first is to include AMD (age-related macular degeneration) and glaucoma in the detection scope to improve the ophthalmic disease matrix; the second is to explore the application of retinal imaging in the early detection of Alzheimer's disease - studies have shown that changes in the retinal nerve fiber layer may be related to the pathological process of Alzheimer's disease, which provides a new path for early diagnosis of the disease.
"The retina is the only part of the human body that can directly observe nerves and blood vessels, and its potential has not yet been fully tapped," said the Ainnova technical team.At present, the retinal feature extraction model for Alzheimer's disease has entered the data accumulation stage and may become another important early diagnosis tool after brain imaging in the future.
Final Thoughts
Starting from the "window" of the retina, Ainnova is redefining the paradigm of preventive medicine: when fundus examinations can simultaneously warn of the risks of diabetes, heart disease, and liver disease, and when pharmacies become "health screening stations," the focus of medical care is shifting from "disease treatment" to "risk prevention."As its mission states, "to make the retina an emerging vital sign" - this is not only a technological vision, but also a practice of "health equality".
Currently, Vision AI's global clinical registration and localized iterations are accelerating. With the advancement of FDA clinical trials, this AI tool, born from the wave of technological innovation, may write the story of "Technology for Good" on a broader stage.
References:
1.https://ir.avanttechnologies.com/press-releases/2024/avant-technologies-and-ainnova-tech-form-joint-venture-to-advance-early-disease-detection-using-artificial-intelligence
2.https://www.itek.cr/post/ainnovatech-and-itek-team-up-to-create-ai-powered-affordable-imaging-device-for-early-disease-detection
3.https://www.biospace.com/press-releases/avant-technologies-and-jv-partner-ainnova-complete-pivotal-meeting-with-u-s-fda